Job opportunity: lab technician
ETAP-Lab is seeking for a laboratory technician for its STROK@LLIANCE in vivo lab in Caen (14000). Job: laboratory technician Type of contract: temporary position (CDD) Duration: 6 months (potentially extended) Under the supervision of the project manager you will contribute to the set up and performing of in vivo and in vitro experimentations and you will ensure proper lab functioning; both in compliance with the Good Laboratory Practice. You have now the opportunity to join a young and dynamic team looking for challenge in a
Clinical evidence confirms Pep2Dia effects on postprandial glycemia in prediabetic subjects
ETAP-Lab had an instrumental position in Ingredia's projects and successfully predicted in mice the efficacy of Pep2Dia, a new bioactive ingredient expected to reduce postprandial blood sugar levels. Ingredia presented at Vitafoods (2019, 8th May 2019, Geneva) clinical evidence that Pep2Dia decreases postprandial blood sugar levels in a prediabetic population. The clinical studies were performed after ETAP-Lab showed a significant benefit of Pep2Dia. Indeed, when administered 15 minutes before a sugar shot in db/db
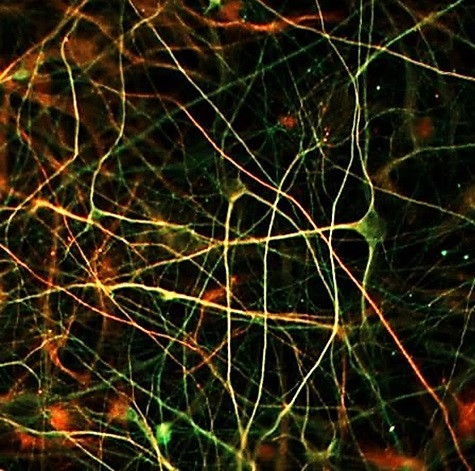
ETAP-Cell: New in vitro services in Neurology
ETAP-Lab launches a new in vitro pharmacology activity in the field of proteopathic neurodegenerative diseases ETAP-Lab proudly announces the launch of ETAP-Cell: a new in vitro pharmacology activity in the field of proteopathic neurodegenerative diseases. The creation of this activity has been made possible by the recruitment of Dr Ahmad Allouche (former Head of Biology at SynAging) who is placing his unique expertise in the solubilization and stabilization of oligomeric proteins (AβO, αSO and TauO) at the disposal of ETAP-Lab. As
Annual Report of 2018
This year was very exciting for ETAP-Lab and we would like to share with you some of the 2018 major achievements: A new atopic dermatitis model, the DNCB-induced model in Balb/c mice, has been developed with effective studies already in progress. New studies for the account of TEMESIS have been performed showing remarkable effects of their lead TEM1657 drug candidate on Psoriasis and results have been presented in different congresses. Different formulations of their product remaining to be characterized. Jean-François
ETAP-Lab participation at the World Preclinical Congress Europe 2018 in Lisbon
ETAP-Lab will be present at the 3rd Annual World Preclinical Congress Europe in Lisbon on November 27-30th. If you are searching for a reliable partner for in vivo preclinical studies, please contact Jean-François BISSON at: jfbisson@etap-lab.com and you could meet there at the end of November. For more information about the meeting click here.
ETAP-Lab has been upgraded to the ISO 9001 certification (2015 Edition)
ETAP-Lab is pleased to announce the upgrade of the ISO 9001 certification, this time based on the latest version of the standard from 2015. Through an internal and external Audit of the OFC (the French Certification Organization): quality assurance, control and monitoring procedures have been carried out to attest to the quality of our services. By being accredited, our company is insuring the customers to meet their expectations by the quality of its management system in the following areas: consulting, advising and expertise of studies
18th Global Dermatology Congress October 25-26, 2018, Budapest, Hungary
Jean-François Bisson, Head of the Department of Dermatology at ETAP-Lab, was present last week at the 18th Global Dermatology Congress in Budapest, Hungary. Researchers from Europe but also from other non-European countries (USA, India, Jordan, Indonesia…) participated to this congress as well. Therefore, most recent advances have been presented concerning the diagnosis and the treatment of several pathologies including wound healing, psoriasis, atopic dermatitis, acne, facial rejuvenation and skin bleaching. A focus on the use of bioengineered skin for preclinical and clinical
Young researcher permanent position on neurodegenerative diseases
The position: ETAP-Lab invites applications for an exciting young researcher permanent position in research and development of a new models in neurodegenerative disease. The successful candidate will work in a collaborative research environment with academical interactions. The first two years, the candidate will be dedicated to the project BIOPROLOR2*; it is a public-private consortium which aims at discovering new plant-derived molecules with health benefits to speed up the development of innovative drugs and active ingredients. ETAP-Lab leads the strategic axis of
STROK@LLIANCE: a new service
ETAP-Lab (Dr Nicolas Violle, President and CEO) is a well-established CRO, with more than 25 years of market knowledge. ESRP is the offshoot of the highly-recognized INSERM unit “Physiopathology and Imaging of Neurological Disorders” (PhIND; Pr. Denis Vivien, Head of the unit). The new platform STROK@LLIANCE is fully dedicated to preclinical stroke in order to address mechanisms, diagnostics and therapeutics in stroke. STROK@LLIANCE manages projects in the field of stroke for the pharmaceutical industry with high-skilled specialists ensuring expertise and creativity to provide high-level animal studies in a