B2B meetings at BIO-Digital: A fast-paced week filled with opportunities!
Thanks to everyone we met - we really appreciated the quality of our interactions, and are as excited as ever to be working alongside you on your preclinical projects. Thanks also to Business France and CCI France, for supporting the export of innovative French biotechs and CROs.
Meet us at BIO-Digital, 8-12 june
ETAP-Lab will participate at Bio-Digital next 8-12 June. Our experts Dr. Jean-François BISSON (VP) and Dr. Nicolas Violle (CEO) will present ETAP-Lab’s services in the fields of preclinical Dermatology and Central Nervous System. Please feel free to reach out if you want to schedule a one-to-one meeting. Looking forward to meeting with you.
COVID-19: Business Continuity
We hope that you are not being too hard hit by the current crisis, and that you and your family and friends remain healthy. Like many others, we have reorganized our production schedule to ensure continuity of service while keeping our team safe. Although things may take a little longer, our team is standing by to conduct your studies to the high-quality standards that underpin our reputation. This period of uncertainty also offers an opportunity for us to reflect on our R&D
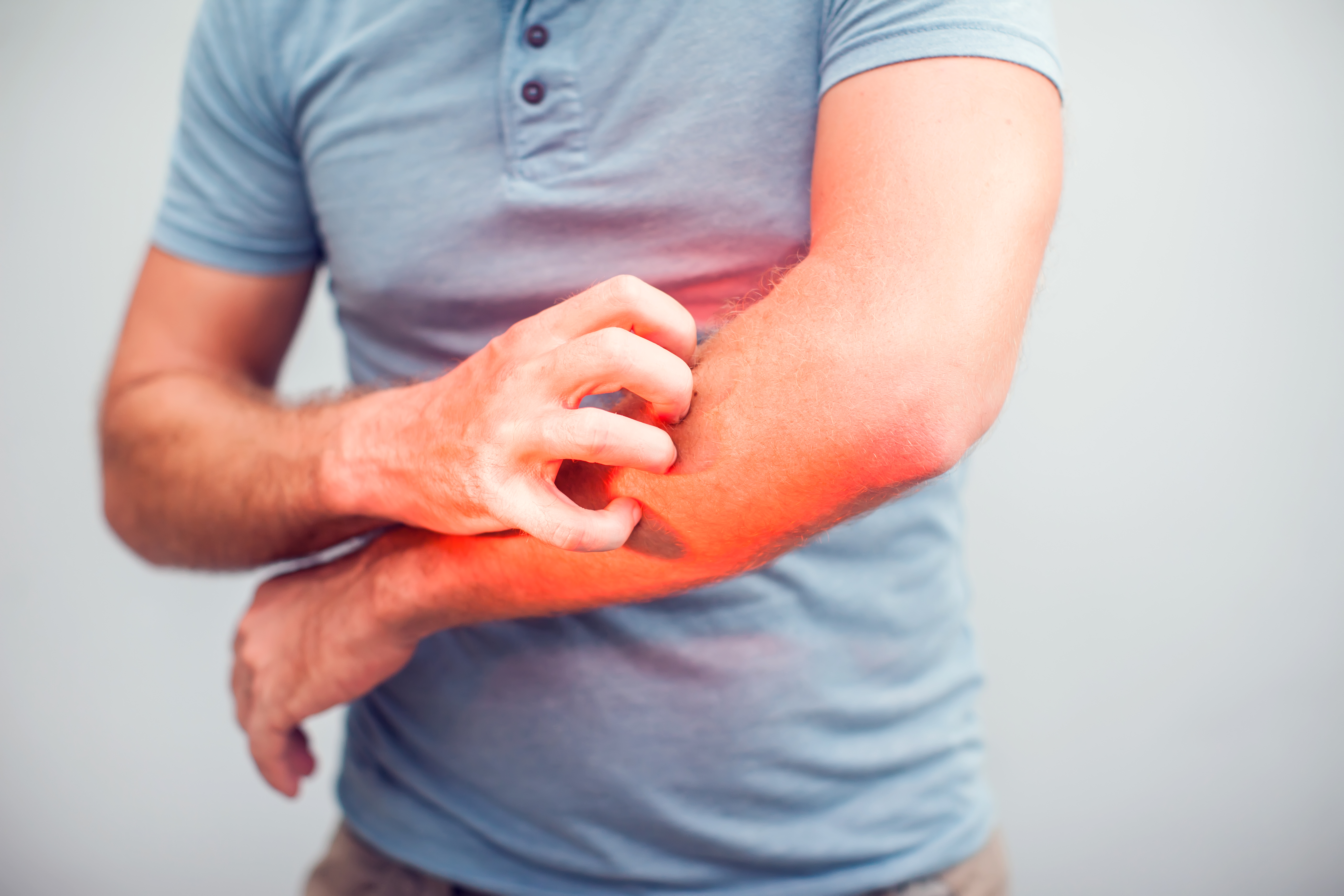
ETAP-Lab’s dermatology activity keeps growing: our first project with a US-based company
By entrusting development of its drug candidate to our expertise, a NASDAQ-listed North American company is following the lead of European and Asian businesses by choosing ETAP-Lab’s DNCB-induced atopic dermatitis mouse model. Indeed, ETAP-Lab has just signed a major contract to assess the effects of a dermatological pharmaceutical treatment currently in development. Atopic dermatitis is a common skin condition that mainly results in redness, lesions and intense itching (pruritus). For patients, it can affect everyday life severely enough to cause anxiety and
STROK@LLIANCE – now a registered trademark in Japan
Created in 2017, the STROK@LLIANCE brand has quickly built a reputation for quality and success in the field of preclinical services in neurology. It is a real pleasure for us to see our brand building value. Having already secured trademark protection in Europe, ETAP-Lab is proud to announce that the Japanese patent office has now approved our application to register the STROK@LLIANCE trademark. We decided to include an image of the official document because, in addition to its legal value, it is beautifully
Nexira invites trusted service provider ETAP-Lab to its inavea™ launch
ETAP-Lab was honoured to be among Nexira’s guests last week at the Printemps Haussmann Coupole in Paris, attending the launch party for its new inavea™ range - the first sustainable, organic ingredients brand with distinctive and proven health benefits. Starting in 2020 with inavea pure acacia, an all-natural dietary fiber sourced from carefully selected acacia trees, Nexira offers a high digestive tolerance and a prebiotic effect. Preclinical studies conducted by ETAP-Lab have proven the health benefits of other products on offer from
Meet us at MEDICA in Düsseldorf, 19 November
ETAP-Lab will participate at MEDICA next 19 November in Dusseldorf. Our experts Dr. Jean-François BISSON (VP) and Dr. Nicolas Violle (CEO) will present ETAP-Lab’s services in the fields of preclinical Dermatology with our partner CYCERON. ETAP-Lab has a track-record of success in the testing of medical devices in the field of Dermatology, such as dressing, plaster and bandage. Please feel free to contact us if you want to schedule a one-to-one meeting. Looking forward to meeting with you. Contact us
Meet us at BIO Europe in Hamburg, 11-13 November
ETAP-Lab will participate at Bio Europe next 11-13 November in Hamburg. Our experts Dr. Jean-François BISSON (VP) and Dr. Nicolas Violle (CEO) will present ETAP-Lab’s services in the fields of preclinical Dermatology and Central Nervous System. Please feel free to contact us if you want to schedule a one-to-one meeting. Looking forward to meeting with you.
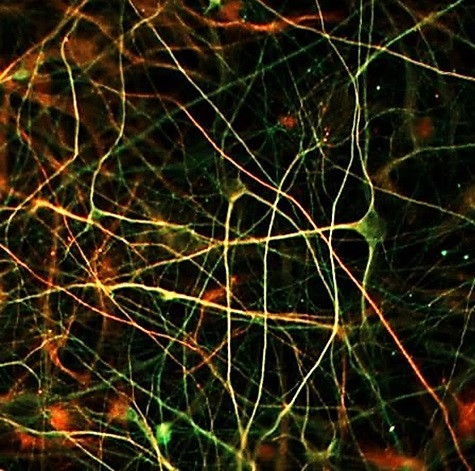
In vitro services are ready to test your drugs for neurodegenerative diseases!
Last June, we produced the 1st batch of beta-amyloid1-42 oligomers in our new facilities, using our unique know-how for oligomers production and stabilization (see here). We are very happy and proud to announce that we have now extensively validated the following in vitro pharmacological models: A model of Alzheimer’s disease: challenge with beta-Amyloid1-42 oligomers in rat primary cortical neuron culture; A model of Parkinson’s disease: challenge with alpha-synuclein oligomers in rat primary cortical neuron culture. Batch-to-batch oligomer production reproducibility has been controlled, confirming the